Building healthcare solutions that are patient centric and personalized.
Life is awesome when the population is healthy and there is an implied need for healthcare to transcend to a patient-centric, personalized, accessible and affordable solutions. Companies leverage our technology partnership and domain understanding of the challenges and complexities of the industry as well as the technologies needed to build the solutions. Our services and solutions encompass a range of business areas, from designing to building solutions that help our clients improve their competitiveness and effectiveness, lower cost of operations, and revitalize operational effectiveness.
Life is awesome
Zool Advantage.
Our IT leadership team has a cumulative experience of over 20 years in working with global life science companies, in diverse geographies. Zool’s Life Sciences practice offers a wide range of innovative solutions for effectively managing drug discovery and development processes. Our objective is to enable and enhance the drug discovery and development cycle by improving productivity while reducing the cost and time-to-market through Life Sciences Consulting Services & Solutions. A strong set of solutions to this critical phase of the lifecycle contributes directly to our ability to understand end-to-end Life Sciences solutions from the basic level. This in turn acts as a baseline to address all other transformational solutions.
Some of the key advantages of Zool are:-
- Zool has a strong team of IT professionals involved in development of solutions in the Pharmacovigilance area. Coupled with the depth in domain knowledge and exposure to various systems in the Pharmacovigilance space.
- Zool’s strong knowledge of the regulatory landscape allows us to recommend appropriate and compliant solutions that meet business needs and systems that are audit-ready.
- Our consultants understand the important interdependencies that exist between safety systems, business processes and compliance.
- Our knowledge of Virtualization tools, multi tenancy concepts and implementation of safety systems gives us the edge for hosting / on boarding customers on safety databases
- Professionals proficient in the leading Safety systems
- Zool has a global delivery model to serve clients in the US, Europe and Asia Pacific
Zool Life Science Services landscape
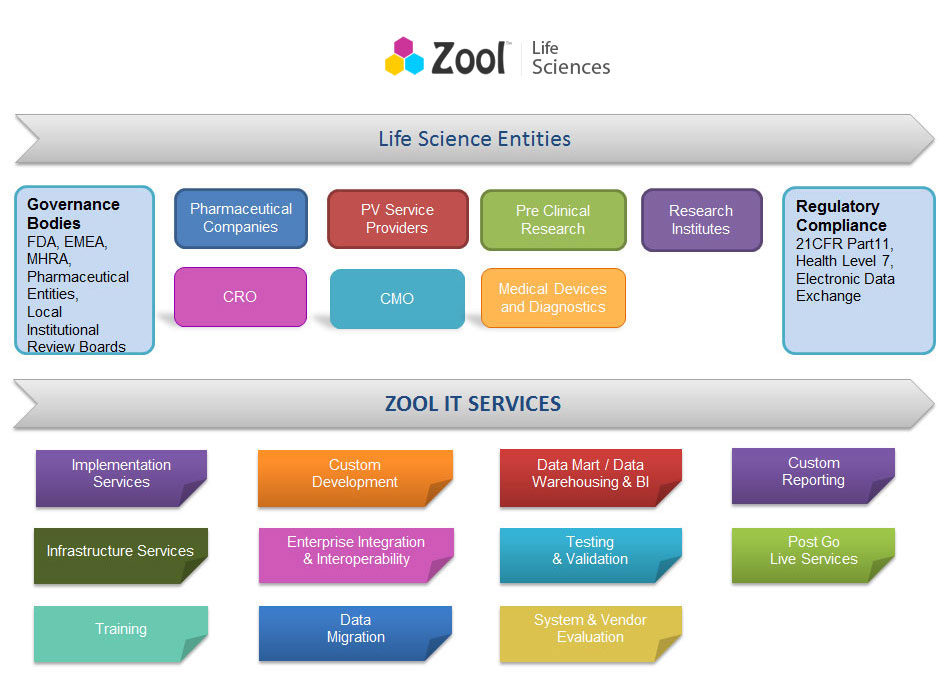
Segments Zool Services
Clinical Development
Leveraging Strategic Partnerships to Transform Clinical Operations. We are the go-to global strategic partner to life sciences companies seeking to accelerate their transformation efforts, improve clinical outcomes and achieve greater levels of cost-efficiency, agility, flexibility and global competitiveness.
All of our solutions are delivered by cross-functional teams with capabilities that accelerate the clinical development process across therapeutic areas and phases. Our process, product and technology centers of excellence enable our teams to help you get the most from your technology investments.
Pharmacovigilance
With growing government and public concern over drug safety, pharmacovigilance (PV) has never been more important. To ensure patient safety, minimize costs and ease regulatory compliance, pharmaceutical firms must aggressively detect and manage emerging safety risks. Yet early risk detection can be undercut by manual processes and a lack of integration across information sources.
Today’s zero-tolerance drug safety environment requires pharma firms to adopt new strategies to quickly identify health safety concerns.
You also need more progressive thinking and business-IT models to address evolving issues such as:
- New compliance requirements for risk management
- Pressure to cut drug discovery costs• Maintaining uniformity and consistency across global PV processes
- Integrating safety data from disparate sources
- To help you reduce regulatory and business risks and achieve new levels of business performance, Zool’s offerings leverage our vast experience in drug safety process consulting, IT solutions implementation and PV process outsourcing.
Regulatory Compliances
The regulatory environment for life sciences companies is known for its challenging complexity. And with thousands of new and changed regulations and guidelines released each year, compliance can be a painful and costly ordeal.
Globalization is forcing companies large and small to find more efficient ways of tracking varying regulations and rapid rule changes. And as new technologies and industry standards emerge, regulatory harmonization and compliance management initiatives are driving life sciences companies to build new systems and processes to comply with mounting guidelines.
Given the exorbitant cost of non-compliance, successful life sciences companies need a strong compliance foundation, including compliance with corporate objectives and policies, working procedures and guidelines and regulator’s requirements.
We have a demonstrated track record of helping global top pharmaceutical, biotech and medical device companies audit and maintain regulatory compliance by optimizing costs and improving process efficiencies. Our audits cover process adequacy, maturity, physical infrastructure, information security and IP protection.
Our regulatory compliance portfolio includes:
- Independent validation services: enabling computer systems compliance in optimal time and cost.
- Application development and maintenance services that are fully 21 CFR 11 and CSV compliant.
- Consulting services, including COTS (Off the shelf product) Evaluation for Part 11 compliance, SPRing (Submission Process Reengineering framework), and ReguWatch (e.g., evaluating the impact of global regulatory updates on processes and systems).
Our success results from a clear understanding that regulatory compliance cannot be viewed in isolation but requires a holistic approach. Whether we are developing a regulated application, maintaining the validated state of an application, or executing mission critical business processes, our standard operating procedures facilitate compliance with various industry standards related to process maturity, information security management, FDA guidelines on computer system validation and electronic records and signatures.
Make regulatory compliance more manageable, cost-efficient and water-tight by taking advantage of our integrated portfolio of services.
Future Vision (Microsoft Health)
We do believe that Life Sceience will have a critical role to play in technology and the future of health in general will be technology enabled and the following video is a motivation for us on what the future holds for health intertwined with technology all around.
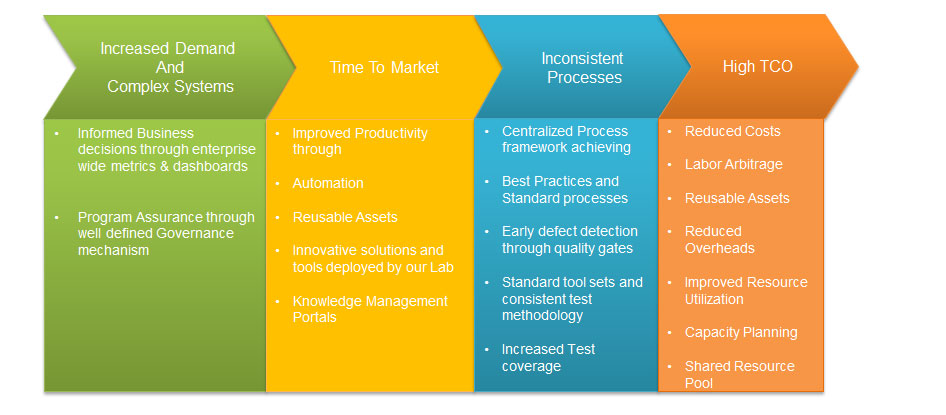
Benefits of Outsourcing
Zool Delivery Model
Zool has a scalable delivery model to cater to the clients in the USA, Europe and Asia Pacific region.
Zool has the classical Onsite / Global Delivery Center (GDC) delivery model where the outsourcing work is distributed between our onsite center and the GDC where the client gets the advantage of both types of outsourcing models. The distribution of work depends on the type of project. Usually 20-30% of the work is done by the onsite center and the rest is done by the GDC.
